US creates strongest-ever armor material with 100 trillion bonds per cm² – Interesting Engineering
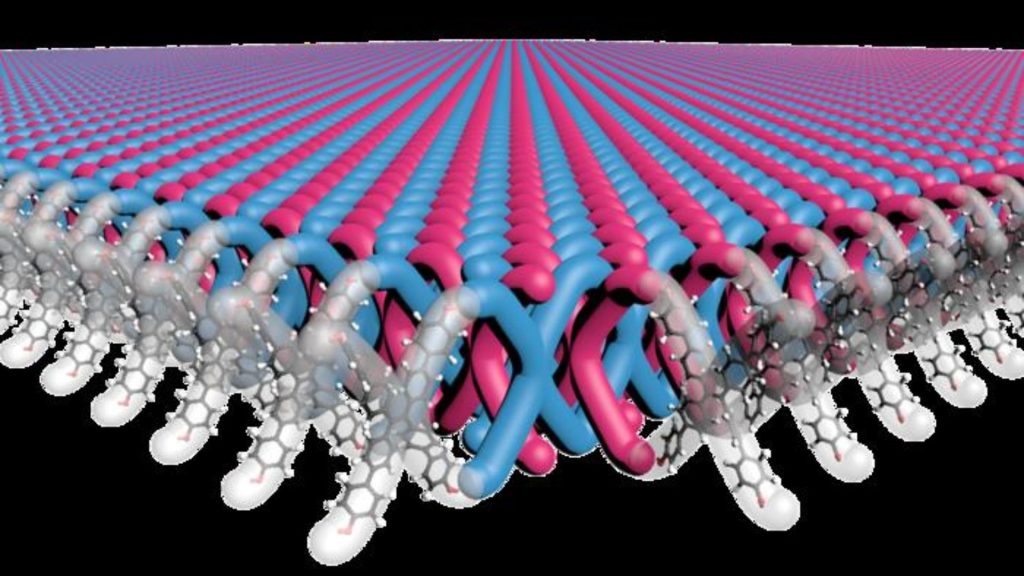
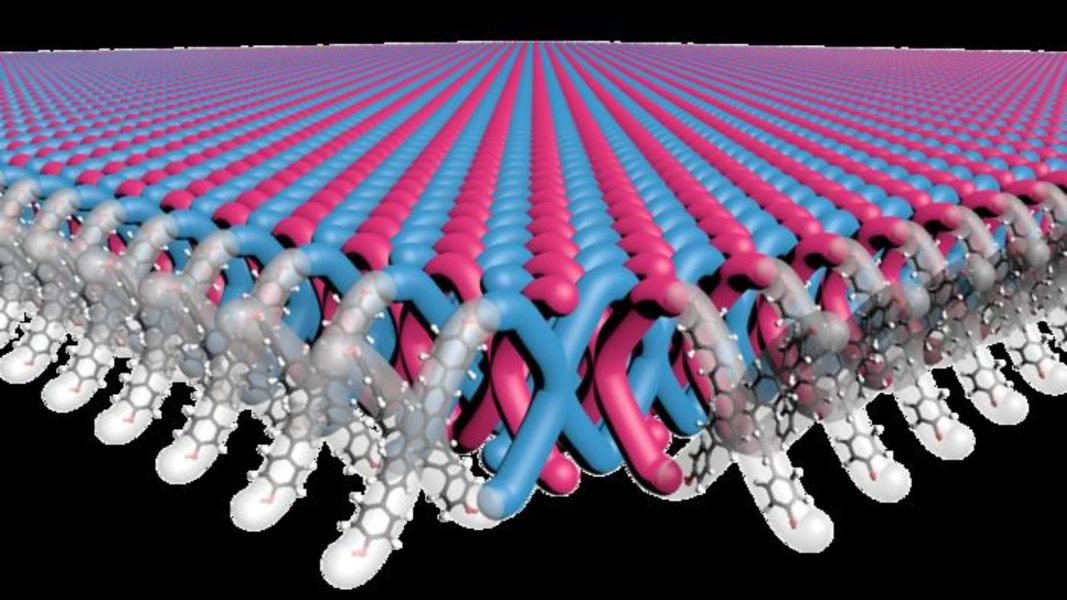
Unlike conventional methods, the manufacturing process of the 2D polymer is highly scalable.16 hours ago19 hours ago19 hours ago20 hours agoa day agoa day ago2 days ago2 days ago2 days ago3 days ago8 hours ago9 hours ago10 hours ago10 hours ago12 hours ago12 hours ago13 hours ago14 hours ago15 hours ago16 hours agoAmeya PalejaIllustration shows X-shaped monomers are interlinked to create the first 2D mechanically interlocked polymer. Mark Seniw, Center for Regenerative Nanomedicine, Northwestern UniversityA research team led by scientists at Northwestern University has developed the first-ever two-dimensional mechanically interlocked material with high flexibility and strength. In the future, this could be used to develop lightweight yet high-performance body armor and other such tough materials, a press release said. It was in the 1980s that Fraser Stoddart, then a chemist at Northwestern University, first introduced the concept of mechanical bonds. Stoddart then expanded the role of these bonds into molecular machines by enabling functions like switching, rotating, contracting, and expanding in multiple ways and using them to develop interlocked structures, which also won him the Nobel Prize in 2016. Researchers have been working on developing mechanically interlocked molecules with polymers for decades but have failed. “In organic chemistry, it is pretty straightforward to form so-called “medium-sized rings” that are 5-8 atoms around. But such rings are too small to thread another molecule through them,” explained William Dichtel, a professor of chemistry at Northwestern University in an email to Interesting Engineering.“In our paper, there are new rings formed at each repeat unit of the 2D structure, which are 40 atoms around,” added Dichtel. This was achieved using an innovative and novel approach that even questioned assumptions about how molecules react. Madison Bardot, a Ph.D candidate in Dichtel’s lab, developed a novel process using X-shaped monomers as building blocks and arranging them into highly ordered crystalline structures. They then used another molecule to create bonds between molecules of the crystal. The resulting material consists of layers of two-dimensional (2D) polymer sheets where ends of X-shaped monomers are interlocked with ends of other X-shaped monomers, and more monomers are threaded through the gaps in between. Together, the material consists of 100 trillion mechanical bonds per square centimeter, the highest density ever achieved. Interestingly, the team also found that dissolving the polymer in the solution allowed the interlocked monomers to peel off each other, enabling the manipulation of individual sheets. “Many highly crystalline substances are brittle, but our polymer has a regular, ordered structure yet is highly flexible because each mechanical bond has a little bit of space to move around,” explained Dichtel in the email to IE. “When one applies a light force to the polymer, it is extremely flexible, but if more force is applied the material becomes more rigid as the mechanical bonds are stretched locally to their limits. This property is called “strain hardening” and is of great interest for ductile and mechanically tough materials.”Beyond mechanical properties, the polymer architecture has interesting properties that can be explored for new applications. Dichtel’s collaborators at Duke University added this newly developed polymer to Ultem, a fiber in the same family as Kevlar but which can withstand extreme temperatures and chemical exposure. Using just 2.5 percent of the polymer dramatically increased its strength and toughness. This could be used for making armor or ballistics protection. While polymers containing mechanical bonds have previously been synthesized at a small scale, this approach helped Dichtel’s team easily make almost a pound (half a kilogram) of the material. This also shows that the approach is highly scalable as well. “Perhaps the most challenging aspect was proving to ourselves that we indeed had the proposed mechanically interlocked structure – it took a team of diverse expertise – synthetic chemists, electron microscopists, polymer engineers – to figure out how to make the materials and then how to actually study them,” added Dichtel in the email. The research findings were published in the journal Science. Stay up-to-date on engineering, tech, space, and science news with The Blueprint.By clicking sign up, you confirm that you accept this site’s Terms of Use and Privacy PolicyAmeya Paleja Ameya is a science writer based in Hyderabad, India. A Molecular Biologist at heart, he traded the micropipette to write about science during the pandemic and does not want to go back. He likes to write about genetics, microbes, technology, and public policy.20 hours ago20 hours agoa day agoa day agoPremiumIE PROFollow
Source: https://interestingengineering.com/science/interlocked-polymer-mechanical-bonds-armor